Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Selenium and Selenoproteins: Characterization, Potentiation, and Intracellular Emerging Roles in Cellular Physiology
*Corresponding author: Emmanuel D Williams, Department of Biology, Livingstone College, Salisbury, North Carolina, USA.
Received: June 07, 2022; Published: June 22, 2022
DOI: 10.34297/AJBSR.2022.16.002256
Abstract
Selenium (Se) is an essential microelement implicated in numerous physiological processes in human health and disease. From physiological roles in thyroid preservation to anti-cancer effects, the role(s) of Se continue to be an intense area of biomedical focus. The roles of Se appear to be, in part, mediated through incorporation of selenium-based proteins termed selenoproteins. In this short review, we review the known roles of these class of proteins, highlighting basic structure, cellular roles, and known implications in health and disease. The overall goal is to provide fundamental appreciation of the importance of selenium and selenoproteins in health and in disease.
Introduction
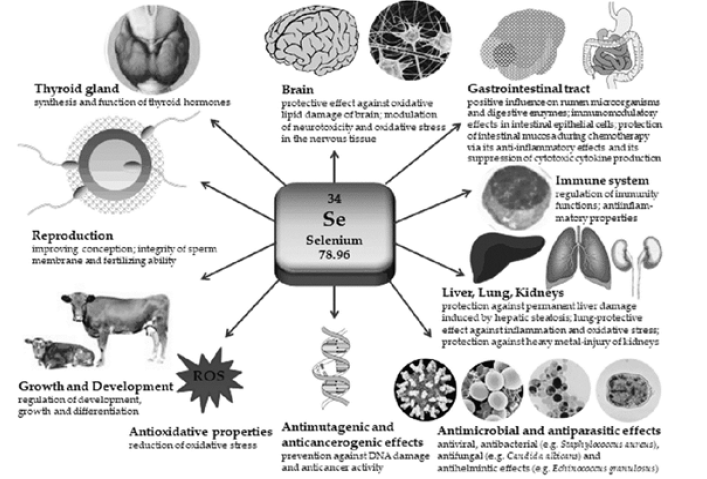
Somatic body cells require adequate nutrition and nourishment for physiological homeostasis. Micronutrients, including trace metals are essential for execution of growth and development, antioxidant effects, and immunocompetency [1]. Selenium (Se) is an essential trace metal that is required to maintain various functions of the body (Figure 1). Se was discovered by Jöns Berzelius in the early 19th century while performing studies on sulfuric acid [2]. Originally thought to be dangerous to humans, advanced chemical analysis in the last 10-15 years has implicated selenium’s roles in various physiological processes [3]. The biological functions of Se appear related to its incorporation through selenocysteine (SeCys) residues into the structure of many proteins important for metabolism [4]. Se exists naturally in both organic and inorganic forms. The organic form is the Se contained in amino acids termed as selenomethionine (SeMet) and SeCys, while the selenate, selenite, selenide, and elemental Se, represent inorganic forms [5]. Inorganically, selenate is the major source of dietary Se in the human population. Through consumption of vegetation and animals, humans typically are exposed to a robust source of selenoproteins (SePs). Selenoproteins have a wide range of cellular functions including selenium transport, co-synthesis and transport of thyroid hormones, cell-mediated immunity, and maintenance of the cellular redox state [6]. Low selenium levels contribute to many diseases including cancer, cardiovascular disease (CVD), and arthritis [7- 10]. Taken together with other physiological outcomes of selenium deficiency, including premature appearance of osteoarthritis (OA), Se appears to link to a physiological phenomenon: cellular aging. The exact contribution of Se to the aging phenotype and more specifically, the premature appearance of age-associated events remains unclear but are almost certainly driven by selenoproteins and selenoprotein-associated factors (Figure 1).
Selenoproteins (SePs): Structure, Function, and Overview
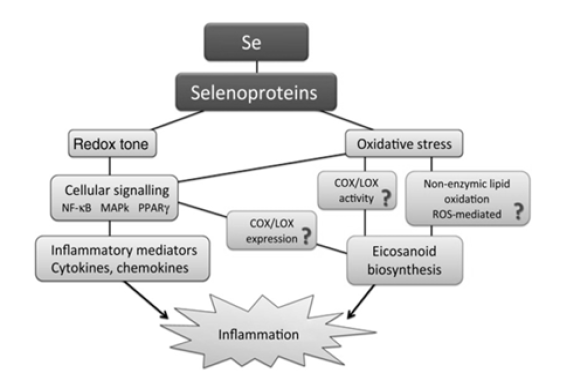
SePs are any protein that includes a selenocysteine amino acid residue [11]. These important molecules and perform critical biological roles, in large part to their anti-inflammatory medication (Figure 2). Dr. Bill Hoekstra first described SePs in the early 1970s through experiments at the University of Wisconsin-Madison [12]. At the present time, over 25 mammalian SePs have been identified from 20-plus genes. From experiments using modern and radioactive analysis, all known SePs are composed of 21 amino acids [13]. The largest class of SePs are involved in cellular antioxidant mechanisms designed at protecting cells against the effects of reactive oxygen and nitrogen species (ROS/RNS). SePs execute their roles intracellularly, primarily as enzyme activators (deiodinases), and as antioxidants such as glutathione peroxidase (GPx). SeP synthesis is essential for Se balance and to protect against many disorders occurring due to disturbance of physiological homeostasis [14]. Two forms of SePs, SeCys and SeMet, are found naturally sources, with SeCys of paramount importance in health and disease [15]. SeCys is found in muscle tissues, poultry, and in Se-containing proteins, whereas SeMet is obtained from various microorganisms and plants [16-17]. Descriptions and clinical correlations about some SePs are described below (Figure 2).
Selenoprotein-P (SelP): Antioxidation and Selenium Transport
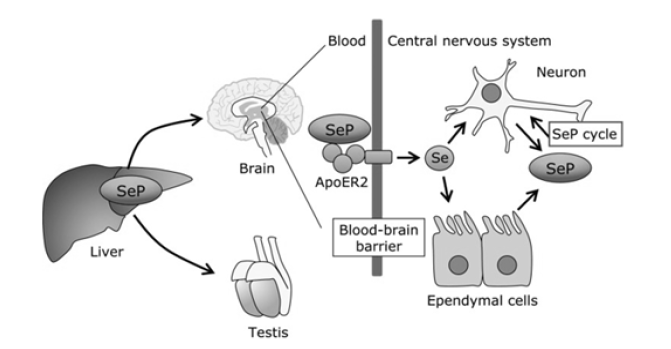
SelP, one of the most widely characterized and abundant SePs, is a glycoprotein first characterized just before the turn of the 21st century [18]. Quantitatively, it accounts for almost half the Se plasma content [19]. Studies have shown that SelP has an important role in selenium synthesis and recycling [20]. Interestingly, Se appears to the signature of SelP deficiency: as Se levels decrease, so do the plasma levels of SelP. Not alarming that reduced SelP levels are also correlated with increased cellular oxidative stress and clinical episodes of disease [21]. Another interesting find showed that in addition to being the most abundant SeP, it appears to be the first (or most sensitive) SeP to increase after Se administration. Only after SelP increase do the other SelPs increase post-Se administration [22]. This seems to suggest that SelP may have a role in the SeP biosynthesis, among its other cellular roles. SelP, and most known SePs, is produced in the liver [23]. SelP consists of two domains: an N-terminal, which assists in maintaining the redox state of the cell, and a smaller C-terminal that acts a Se transporter [24]. SelP expressivity extends to the testes and brain tissues, with reports of expression in the brain and fetal tissue (Figure 3). Physiologically, SelP appears to function in plasma transport of Se within tissues. SelP protects lipoproteins against free radical damage by neutralizing peroxynitrite formed as a byproduct of the reaction between superoxide and nitric oxide species [25,26]. SelPs are also implicated as a source of Se for peripheral tissues [27]. In vivo studies with SelP-knockout mice showed that administration of a normal Se diet showed reduced Se levels in the testes and nervous tissue, whereas Se levels in the kidneys were marginally affected [28] (Figure 3).
Selenoprotein W (SepW): Muscle Physiology
SepW is a 10-kDa protein that is highly expressed in mammals. SepW is a cytosolic protein that is concentrated in the brain and muscle tissue [29]. SepW appears essential for muscle physiology in animals. The complete and definitive cellular role of this SepW is still unclear, but studies have revealed sparse details about this protein. Studies have shown that SepW protein expression levels were increased in animal tissues when Se was increased in the diet [30]. Other clinical and in vivo studies focused on the relationship between SepW and human muscle disease have been done with correlative, but limited findings [31]. Another in vitro study has implicated SepW as a part of the stress-related community of SePs as its increased expression correlated with reduced superoxide formation in high-glucose supplemented cardiomyocytes [32].
Thioredoxin Reductase: Cancer, Cellular Redox and Tumorigenesis
Thioredoxin reductase composes three (3) enzymes: TR1 (thioredoxin reductase 1), TR2, and TR3. Each enzyme functions as all act as independent antioxidant molecules by mediating cellular redox reactions [33]. TR1, TR2, and TR3 also play a role in apoptosis and de novo DNA synthesis [34]. Thioredoxin reductase serves as a functional regulator for the metabolism of Se compounds by providing oxidized selenium (selenide) for SePs synthesis [35]. Thioredoxin reductase is known to phosphorylate and activate the p53 gene, a known tumor suppressor [36]. Consistently, and TR1 expression is increased in many carcinoma cells and tumors, including prostate [37]. In vitro sequestration of TR1 in cancerous cell lines contributed to cellular senescence and reduction of cell cycle progression. Further, in vivo re-administration of TR1- knockdown cells into mice inhibited cell proliferation as compared to a control cell line [38]. From these findings it appears TR1 can exhibit two opposing functions in the pathology of cancer. TR1 can serve as an anti-cancer agent by maintaining the cell redox state, reducing the mutation rate and subsequent tumor development. TR1 is also required for tumorigenesis due to cancer cells are highly sensitivity and vulnerability to oxidative stress [39].
Glutathione Peroxidase (GPx): Antioxidation of Hydrogen Peroxide
Glutathione peroxidase (GPx) group SePs appear in archaea, bacteria, and eukaryotes [40]. While precise GPx proteins haven’t been identified in bacteria and terrestrial plants, DNA sequencing has revealed they contain SeCys-containing GPx sequence homology. GPx proteins perform various physiological roles in cells, most notably the neutralization and detoxification of hydrogen peroxide (H2O2), as well as maintaining the of cell redox state [41]. GPx1 expressivity is ubiquitous throughout the human body, with increased concentration in erythrocytes, parietal epithelial cells of the kidneys, the lungs, and hepatocytes [42]. GPx proteins function primarily as an antioxidant and is believed to be impacted by Se deficiency [43]. One GPx class, GPx2, is expressed in the gastrointestinal (GI) tract where it functions to neutralize free radicals [44]. Another class of GPx proteins, GPx3, is present in various extracellular fluids and is responsible for nearly one-third of the Se levels in the blood [45]. GPx3 has also been found in various organs ranging from the breast to the heart to even the placenta in expecting mothers [46]. GPx3 reduces lipid hydroperoxides production in the blood [47]. GPx4 is also ubiquitous with primary expression in the testes. Intracellularly, GPx4 is nuclear, cytosolic and mitochondrially located [48]. GPx4 is required for sperm development and motility [49]. GPx5, 6, 7, and 8 have been identified, but their functions remain an intense area of research [50].
Discussion
Our understanding of Se and SePs continues to evolve as its importance in health and disease become clear. In addition, Se and SePs are widely express and play important roles ranging from maintenance of the cellular redox state of cells, cellular transport, to even anti-tumorigenesis. Like all molecules, optimum levels are correlated with health and disease outcomes. Se deficiency is correlated with decreased ROS and reactive nitrogen species (RNS) damage and muscle dysfunction in mammals. Elevated levels of Se and SePs drive the development of carcinomas. This highlights one of the critical questions around selenium importance: what are optimum levels? Clinical suggestions of 70- 95 mg/dL in adults remain an area of great debate as outcomes observed with Se deficiency are manifested in European and East Asian demographics who maintain these Se levels. This appears to suggest that environmental and genetic factors may play a role in Se levels and physiology.
Advanced aging and the link between Se-based compounds also needs further elucidation. It is known (and not surprising) that decreased Se levels lead to the premature appearance of biomarkers of senescence in vitro. Oxidative stress, a hallmark of mitochondrial dysfunction, is known to drive cellular aging and selenoproteins are strong antioxidant agents. However, in populations above the age of 65, re-administration of Se restores trace metal levels, but does not equate to incorporation and synthesis of SePs. A few hypotheses exist for this molecular event, but more studies are needed.
References
- Mirnamniha M, Faroughi F, Tahmasbpour E, Ebrahimi P, Beigi Harchegani A (2019) An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev Environ Health 34(4): 339-348.
- Reich HJ, Hondal RJ (2016) Why Nature Chose Selenium. ACS Chem Biol 11(4): 821-841.
- Rayman MP (2012) Selenium and human health. Lancet 379(9822): 1256-1268.
- Arnér ESJ (2020) Common modifications of selenocysteine in selenoproteins. Essays Biochem 64(1): 45-53.
- Spallholz JE (2019) Selenomethionine and Methioninase: Selenium Free Radical Anticancer Activity. Methods Mol Biol 1866: 199-210.
- Köhrle J (2021) Selenium in Endocrinology-Selenoprotein-Related Diseases, Population Studies, and Epidemiological Evidence. Endocrinology 162(2): bqaa228.
- Marciel MP, Hoffmann PR (2019) Molecular Mechanisms by Which Selenoprotein K Regulates Immunity and Cancer. Biol Trace Elem Res 192(1): 60-68.
- Schomburg L, Orho Melander M, Struck J, Bergmann A, Melander O (2019) Selenoprotein-P Deficiency Predicts Cardiovascular Disease and Death. Nutrients 11(8): 1852.
- Turrubiates Hernández FJ, Márquez Sandoval YF, González Estevez G, Reyes Castillo Z, Muñoz Valle JF (2020) The Relevance of Selenium Status in Rheumatoid Arthritis. Nutrients 12(10): 3007.
- Li XX, Guan HJ, Liu JP, Guo YP, Yang Y, et al. (2015) Association of selenoprotein S gene polymorphism with ischemic stroke in a Chinese case-control study. Blood Coagul Fibrinolysis 26(2): 131-135.
- Stadtman TC (1996) Selenocysteine. Annu Rev Biochem 65: 83-100.
- Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284(2): 723-727.
- Yim SH, Tobe R, Turanov AA, Carlson BA (2018) Radioactive 75Se Labeling and Detection of Selenoproteins. Methods Mol Biol 1661: 177-192.
- Liu J, Cheng R, Rozovsky S (2018) Synthesis and semisynthesis of selenopeptides and selenoproteins. Curr Opin Chem Biol 46: 41-47.
- Barceloux DG (1999) Selenium. J Toxicol Clin Toxicol 37(2): 145-172.
- Guo L, Yang W, Huang Q, Qiang J, Hart JR, et al. (2018) Selenocysteine-Specific Mass Spectrometry Reveals Tissue-Distinct Selenoproteomes and Candidate Selenoproteins. Cell Chem Biol 25(11): 1380-1388.
- Mangiapane E, Pessione A, Pessione E (2014) Selenium and selenoproteins: an overview on different biological systems. Curr Protein Pept Sci 15(6): 598-607.
- Tsutsumi R, Saito Y (2020) Selenoprotein P; P for Plasma, Prognosis, Prophylaxis, and More. Biol Pharm Bull 43(3): 366-374.
- Lamarche J, Ronga L, Szpunar J, Lobinski R (2021) Characterization and Quantification of Selenoprotein P: Challenges to Mass Spectrometry. Int J Mol Sci 22(12): 6283.
- Shetty S, Copeland PR (2018) Molecular mechanism of selenoprotein P synthesis. Biochim Biophys Acta Gen Subj. 1862(11): 2506-2510.
- Saito Y (2020) Selenoprotein P as an in vivo redox regulator: disorders related to its deficiency and excess. J Clin Biochem Nutr 66(1): 1-7.
- Saito Y (2020) Selenoprotein P as an in vivo redox regulator: disorders related to its deficiency and excess. J Clin Biochem Nutr 66(1): 1-7.
- Zhang Z, Guo Y, Qiu C, Deng G, Guo M (2017) Protective Action of Se-Supplement Against Acute Alcoholism Is Regulated by Selenoprotein P (SelP) in the Liver. Biol Trace Elem Res 175(2): 375-387.
- Burk RF, Hill KE (2015) Regulation of Selenium Metabolism and Transport. Annu Rev Nutr 35:109-134.
- Jin Y, Chung YW, Jung MK, Lee JH, Ko KY (2020) Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes. Cell Mol Life Sci 77(12): 2367-2386.
- Takamura T (2020) Hepatokine Selenoprotein P-Mediated Reductive Stress Causes Resistance to Intracellular Signal Transduction. Antioxid Redox Signal 33(7): 517-524.
- Moschos MP (2000) Selenoprotein P. Cell Mol Life Sci. 57(13-14): 1836-1845.
- Chen LL, Huang JQ, Xiao Y, Wu YY, Ren FZ, et al. (2020) Knockout of Selenoprotein V Affects Regulation of Selenoprotein Expression by Dietary Selenium and Fat Intakes in Mice. J Nutr 150(3): 483-491.
- Whanger PD (2000) Selenoprotein W: a review. Cell Mol Life Sci 57(13-14):1846-1852.
- Shin MG, Cha HN, Park S, Kim YW, Kim JY, et al. (2019) Selenoprotein W deficiency does not affect oxidative stress and insulin sensitivity in the skeletal muscle of high-fat diet-fed obese mice. Am J Physiol Cell Physiol 317(6): C1172-C1182.
- Jeon YH, Park YH, Lee JH, Hong JH, Kim IY (2014) Selenoprotein W enhances skeletal muscle differentiation by inhibiting TAZ binding to 14-3-3 protein. Biochim Biophys Acta 1843(7): 1356-1364.
- Liu W, Yao H, Zhao W, Shi Y, Zhang Z, et al. (2016) Selenoprotein W was Correlated with the Protective Effect of Selenium on Chicken Myocardial Cells from Oxidative Damage. Biol Trace Elem Res171(2): 419-426.
- Arnér ES, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267(20): 6102-9.
- Joardar N, Sen A, Rath J, Babu SPS (2021) Inhibition of thioredoxin reductase (TrxR) triggers oxidative stress-induced apoptosis in filarial nematode Setaria cervi channelized through ASK-1-p38 mediated caspase activation. Mol Biochem Parasitol. 242: 111364.
- Lu J, Berndt C, Holmgren A (2009) Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim Biophys Acta 1790(11): 1513-1519.
- Abdelwahab EMM, Bovari Biri J, Smuk G, Fillinger J, McPhail D, et al (2021) Activated p53 in the anti-apoptotic milieu of tuberous sclerosis gene mutation induced diseases leads to cell death if thioredoxin reductase is inhibited. Apoptosis 26(5-6): 253-260.
- Singh SS, Li Y, Ford OH, Wrzosek CS, Mehedint DC, et al. (2008) Thioredoxin Reductase 1 Expression and Castration-recurrent Growth of Prostate Cancer 1(3): 153-157.
- Poerschke RL, Moos PJ (2011) Thioredoxin reductase 1 knockdown enhances selenazolidine cytotoxicity in human lung cancer cells via mitochondrial dysfunction. Biochem Pharmacol 81(2): 211-221.
- Arnér ESJ (2017) Targeting the Selenoprotein Thioredoxin Reductase 1 for Anticancer Therapy. Adv Cancer Res 136: 139-151.
- Flohé L (1988) Glutathione peroxidase. Basic Life Sci 49: 663-668.
- Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5(1): 51-66.
- Cheng Y, Xu T, Li S, Ruan H (2019) GPX1, a biomarker for the diagnosis and prognosis of kidney cancer, promotes the progression of kidney cancer. Aging (Albany NY) 11(24): 12165-12176.
- Tian R, Geng Y, Yang Y, Seim I, Yang G (2021) Oxidative stress drives divergent evolution of the glutathione peroxidase (GPX) gene family in mammals. Integr Zool 16(5): 696-711.
- Wingler K, Brigelius Flohé R (1999) Gastrointestinal glutathione peroxidase. Biofactors 10(2-3): 245-249.
- Chang C, Worley BL, Phaëton R, Hempel N (2020) Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers (Basel). 12(8): 2197.
- Zhao H, Kalish FS, Wong RJ, Stevenson DK (2018) Hypoxia regulates placental angiogenesis via alternatively activated macrophages. Am J Reprod Immunol 80(3): e12989.
- Nirgude S, Choudhary B (2021) Insights into the role of GPX3, a highly efficient plasma antioxidant, in cancer. Biochem Pharmacol 184:114365.
- Forcina GC, Dixon SJ (2019) GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 19(18): e1800311.
- Fallahi S, Rajaei M, Hesam MJ, Koolivand M, Malekzadeh K (2021) The effect of Phoenix dactylifera pollen on the expression of NRF2, SOD2, CAT, and GPX4 genes, and sperm parameters of fertile and infertile men: A controlled clinical trial. Int J Reprod Biomed (6): 545-558.
- Ahmed Khan Z, Mishra C, Jyotiranjan T (2020) In silico analysis of caprine superoxide dismutase 1 (SOD1) gene. Genomics (1): 212-217.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.